ABOUT COMPANY
A few words about us

SoftSystem operates according to clearly defined values: dedication and commitment to achieve the best possible results, trust, openness and professionalism. More than 30 years of successful implementations and projects have allowed us to gain confidence and experience. We are constantly evolving, seeking innovative solutions to meet the diverse needs and requirements of our clients.

SoftSystem is one of the leading companies in the European market for the development and operation of laboratory IT systems and integration services for medical facilities operating worldwide. Founded in 1990, the company has grown rapidly and is gaining an excellent reputation as a valued employer and reliable software provider.
OUR PURPOSE
What do we specialise in?
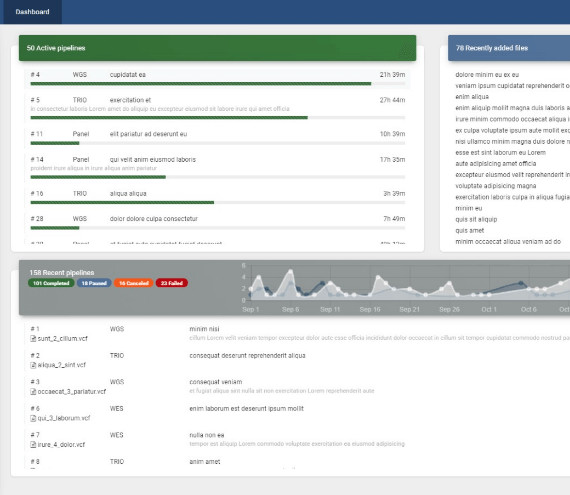
Development and operation of laboratory information systems
30+
Years of experience
With more than 30 years of successful implementations and projects, we have gained confidence and experience. We are constantly evolving, looking for innovative solutions to meet the diverse needs and requirements of the global medical institutions
300+
Systems Implemented Worldwide
Customer satisfaction and the trust developed over many years is the source of our company's success
550+
programmers and testers
Our employees are our greatest asset. We select them on the basis of a carefully prepared recruitment process that promotes knowledge and commitment
PRODUCTS
Our other systems

QMS
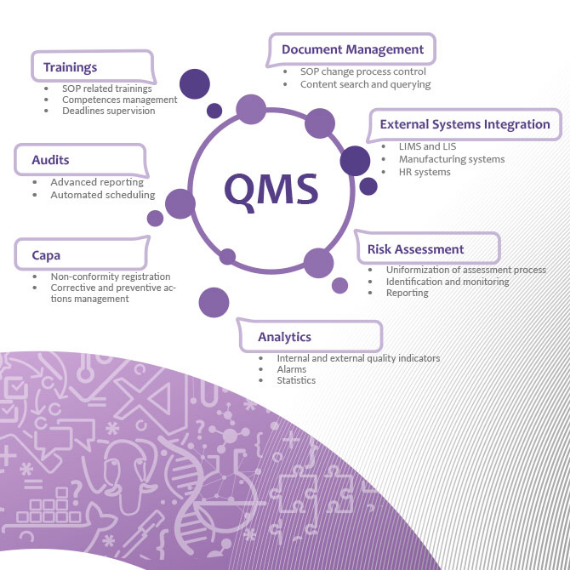
Quality Management System
An application for effective document management in a way that supports the processes of the quality management system processes. Documents such as the Quality Manual, General Procedures, Standard Operating Procedures, Instructions Operational Procedures, Instructions, together with their supporting documentation are stored and processed in the system.
The processes of creating, reviewing and approving changes to the quality system are handled in the application, completely eliminating the need to store paper versions of documents. As part of the handling of the quality management system, it is also possible to document corrective and remedial actions, potentials for improvement, risk management and planning and conducting audits.
The application allows integration with external systems, such as laboratory management systems or production systems, so that we can clearly and transparently monitor the functioning of all aspects of our organisation's quality management system.

BioBank
Software for managing sample repositories
SoftBiobank is a software developed for the management of sample repositories, allowing the annotation, storage and supervision of the distribution of biological materials.
The system enables scientific and commercial projects while ensuring data security and compliance with all biobanking regulations.
The ease of searching the collected resources and the extensive reporting capabilities make the SoftBiobank is an ideal solution for clients collecting large collections of samples, not limited to banking a single type of material.

Data portal
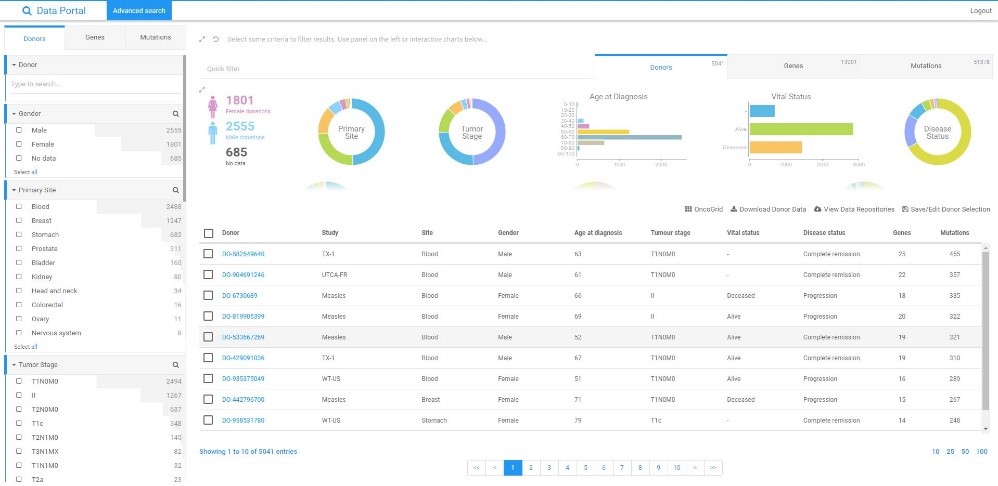
Software for managing sample repositories
Data portal is a platform designed to store, publish and share data from different applications and from different users. This makes data collection easier and more practical. The user-friendly view is designed for quick and intuitive search of large data sets.
The information available in laboratory systems, such as LIMS, is presented in a process-oriented and patient/report-oriented. Specialised search capabilities allow access to a specific patient/case.
The Data Portal is designed for professionals who need a comprehensive overview of the of collected data. The data is presented mainly on a cohort basis with the possibility of advanced searching even very large collections.

NGS Console
NGS Data Processing System
NGS-Console software is dedicated to laboratories performing next-generation sequencing generation (NGS) sequencing. It integrates a variety of tools and packages, including GATK, Picard, SAMTools, FASTQC into a one simple and easy-to-use automated framework.
The modular architecture of the system allows the incorporation of alternative, commercially available packages and open-source bioinformatics algorithms.
Each stage of data processing can be supervised in real time, providing full control over the process. In addition, users have access to a variety of output files, including the BAM file, BAI file, VCF file and final CVS report.